Hotline
Principle of percutaneous Kyphoplasty Procedure
Percutaneous Kyphoplasty Procedure is performed under the monitoring of image-enhanced assembly, percutaneous puncture to the compressed centrum to eastablish a working channel. A special-made balloon of about 15mm in size is delivered to the center of the centrum, and then injects the contrast agent into the balloon to expand the balloon, gradually support the collapsed centrum, and partially or completely restore the height of the centrum. The balloon is finally removed and the bone cement is filled into the "cavity" of the center of the centrum through the working channel. After the operation, the pan can be quickly relieved; the height of the centrum can be restored; the kyphosis can be corrected; the fracture can be restored and stabilized; and the patients' life quality can be improved.
Surgical Procedures

(1) Percutaneous puncture under the perspective guidance, drill "vertebra perforator" into the upper margin of external border of pedicle shadow.
Note: When the "vertebral perforator needle tip" to the posterior wall of the vertebral body, the positive side perspective needle tip is located in the medical margin of the pedicle. Under side perspective, continue to drill into 2-3mm.
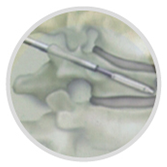
(2) Draw out the needle body in the vertebral perforator, insert the "guide needle" along the "vertebral perforator casing", and pull out the "vertebral perforator casing".
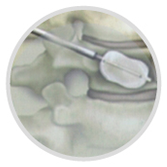
(3)Insert the "dilator assembly" along the "guide needle" to 2-3mm in front of the posterior cortex of the vertebral body, remove the "dilator" and "guide needle", and retain the "dilated casing" as a working channel.

(4)Place "hand drill" along the working channel and slowly drill into the vertebral body with the strength of the finger.
Note: Under perspective, the lateral position should show that the tip of the drill bit has reached the anterior edge of the vertebral body, and the positive position should show that the hand drill head part is near the edge of the spinous process.
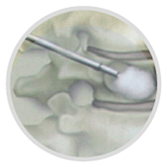
(5)Remove "hand drill" and place "Kyphoplasty Balloon Kit". Under perspective, expand the balloon slowly to restore the vertebral height and create a cavity in the vertebral body.
Note: When the pressure reaches 50 PSI, remove the "strengthened needle" and the maximum pressure to the end plate, or the height of the vertebral body is satisfactory, or if the pressure builds up and cannot continue, balloon dilation should be stopped.

(6)Remove the balloon and inject the finished bone cement the "cement delivery tube" and use the "introducer stylet" to slowly push the bone cement into the vertebral body cavity.

(7)Slowly remove the dilated casing. Close the wound with the sterile wound plast and complete the procedure.
Patient position
Place the patient in any position that can reduce the load on the fractured vertebral body. If the fracture is on the curvature of the lumbar lordosis of the spine, place the patient in the spine hyperextension position. The operating table should be radiolucent to allow the C-arm machine to be used for frontal and lateral observation of the patient.

Characteristic
● Minimally invasive surgery can reduce tissue damage and bleeding volume;
● Surgical operation is simple and quick, when greatly shortens the operation time;
● Local anesthesia can be used to reduce the risk of operation and postoperative complications;
● Pain relief immediately after operation, effectively recover the height of the vertebral body and correct spinal kyphosis.
Indications
● Simple fresh spinal thoracolumbar vertebral body compression fractures due to osteoporosis in the middle age with no neurological combined injury;
● Old spinal compression fractures (more than six months), intractable low lumbage and back pain caused by servere kyphosis with fracture;
● Secondary multifractional compression fractures in the upper and lower adjacent vertebrae secondary to osteoporotic compression fractures;
● Pathological compression fracture caused by benign and malignant vertebral body tumors.
Absolute contraindications
● Burst fracture of the vertebral body with a combination of nervous system injuries;
● People with serious dysfunction of heart, liver and kidney who can not endure operation;
● Patients with compression fractures combined with facet jonits or intervertebral joint dislocation;
● Patients with osteomyelitis or systemic infection exists;
● Patients have hyperlipidemia, with history of lower limb or systemic vascular embolism;
● Pregnant woman;
● A person with an allergy to cement or filler.
Other Products







Blon public account

Mobile Website
Blon Medical Technologies
Address: 3230 Fallow Field Dr.Diamond Bar, CA 91765, USA
E-mail: info@blonmedicaltech.com
Tel:909 655-8119
Cooperation Hotline
+86-0519-86470196
Address: No. 525 Changwu Road, Wujin High-tech Development Zone, Changzhou City, Jiangsu Province
Tel:+86-0519-86470196 / 13801500434
E-mail:info@blonmedical.com
Fax:+86-0519-86488930
Copyright © 2019 Changzhou Blon Minimally Invasive Medical Devices Technology Co. Ltd. 苏ICP备19073665号-1









